Do Oral ULTs Reach Goal?
Percentage of patients treated with febuxostat and allopurinol who reached target sUA level2,6
The pooled phase 3 results for the percentage of patients with an sUA level of <6 mg/dL at the final visit for febuxostat 80 mg was 65% and for allopurinol 300 mg was 40%.7,8
If patients fail to reach sUA target levels <6 mg/dL on oral ULTs at maximum medically appropriate doses and continue to have frequent gout flares (>2 flares/year) or nonresolving tophi, gout remains uncontrolled.9
sUA, serum uric acid.
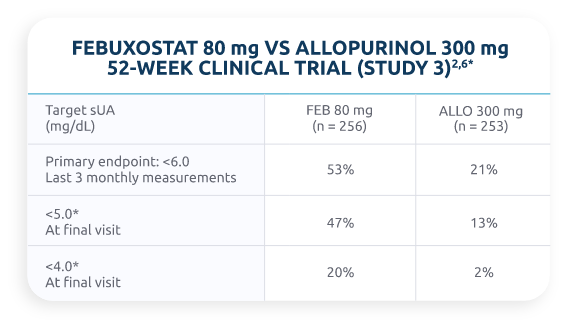
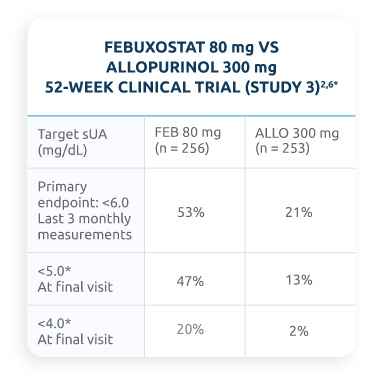
*Post hoc analysis.

